Vivian de Waard
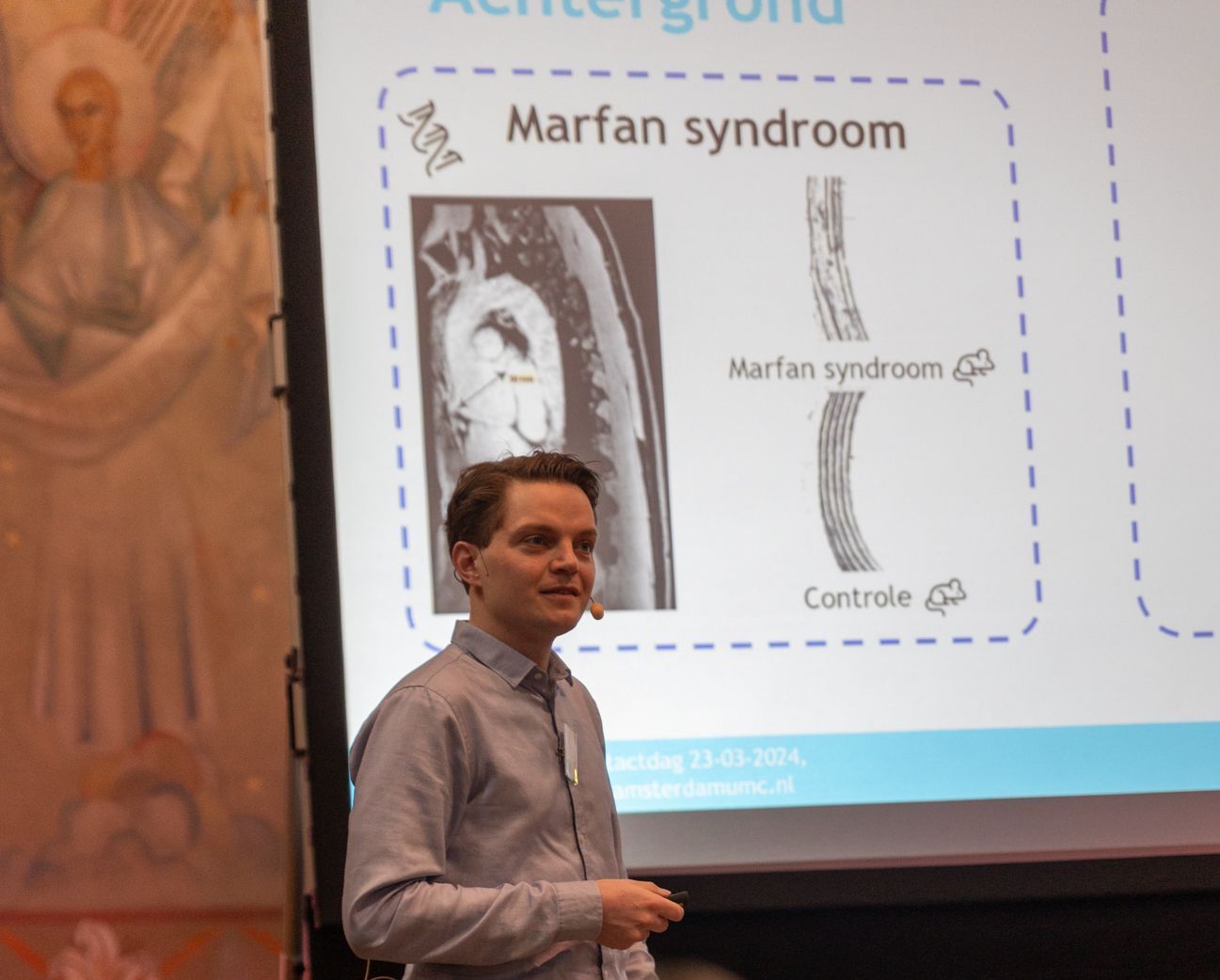
Marfan Syndrome

Vivian de Waard
Associate prof. Vivian de Waard, PhD
Biosketch
Vivian de Waard is trained as a medical biologist at the University of Amsterdam. During her studies she performed internships on (dermal) fibroblasts and endothelial cells. In a year abroad at The Scripps Research Institute in La Jolla (CA, USA), she worked on adipocytes. During her PhD the focus was on smooth muscle cells in restenosis, and in her post doc years she worked on atherosclerosis and aortic aneurysm formation. When she started her own lab at the Medical Biochemistry department, she specialized in genetic aneurysm diseases, mostly Marfan syndrome. Looking back, she worked on all cell types of the vasculature, namely adipocytes, fibroblasts, smooth muscle cells and endothelial cells. The interaction of these cell types with eachother, and the extracellular matrix network they produce, determines how the vascular wall functions and responds to stress. Uncovering novel targets for treatment of vascular diseases lies hidden in resolving this complex vascular environment.
The de Waard-lab aims to unravel the pathological signaling pathways in patients with aortic aneurysm disease and especially in patients with Marfan syndrome by performing fundamental and translational research in disease-specific cell and animal models. The focus is on finding effective pharmacological treatments to prevent or stabilize aortic disease, which is currently lacking.
v.dewaard@amsterdamumc.nl
De Waard Lab
News

De Waard Lab
Research
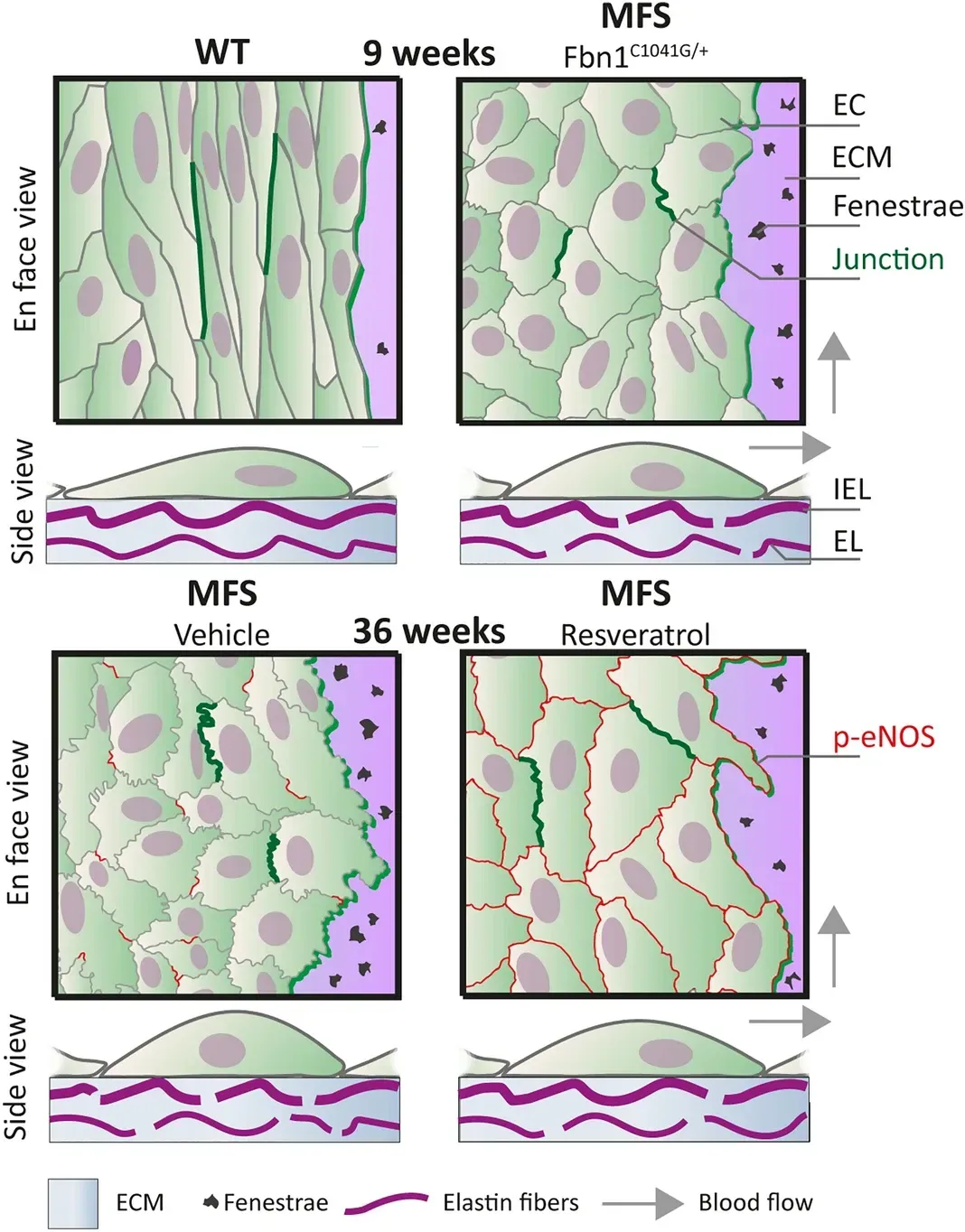
Endothelial dysfunction in Marfan syndrome mice is restored by resveratrol
Using en face immunofluorescence confocal microscopy, we showed that endothelial cell alignment with blood flow was reduced and junctional linearity was decreased in aortae of in Marfan mice (Fbn1C1041G/+). This modified phenotype was most prominent in the ascending aorta and occurred before aortic dilatation. To reverse the diseased endothelial morphology, we performed a 3 week treatment in Marfan mice with polyphenol resveratrol. This restored blood flow alignment, junctional linearity, phospho-eNOS expression at the cell membrane, and improved the structural integrity of the internal elastic lamina of the Marfan aorta.
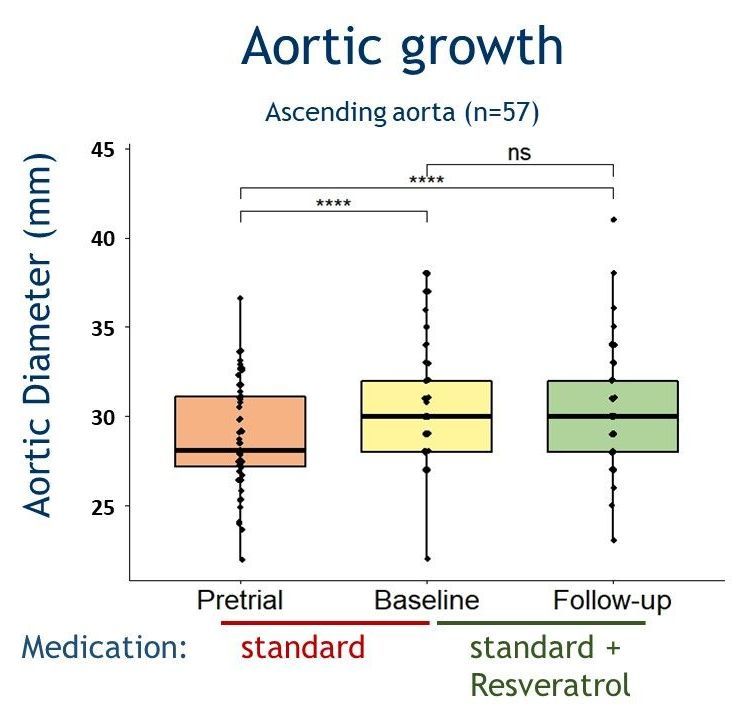
Effects of resveratrol on aortic growth in patients with Marfan syndrome: a single-arm open-label multicentre trial
Fiftyseven Marfan syndrome patients,
aged 37±9 years, of which 28 males (49%) were included in the clinical trial (NL66127.018.18). Approximately half of them, 46% (26/57), had undergone aortic root replacement prior to the study, thus were studied for the remaining thoracic aorta. Aortic root diameters remained stable after 1.2±0.3 years of resveratrol administration. A trend towards a decrease in estimated growth rate (mm/year) was observed in the aortic root (native root only; from 0.39±0.06 to -0.13±0.23, p=0.072), ascending aorta (from 0.40±0.05 to -0.01±0.18, p=0.072) and distal descending aorta (from 0.32±0.04 to 0.01±0.14, p=0.072). Thus, Resveratrol treatment for 1 year may stabilise the aortic growth rate in adult patients with Marfan syndrome. However, a subsequent randomised clinical trial with a longer follow-up duration and a larger study cohort is needed to establish an actual long-term beneficial effect of this dietary supplement in patients with Marfan syndrome.
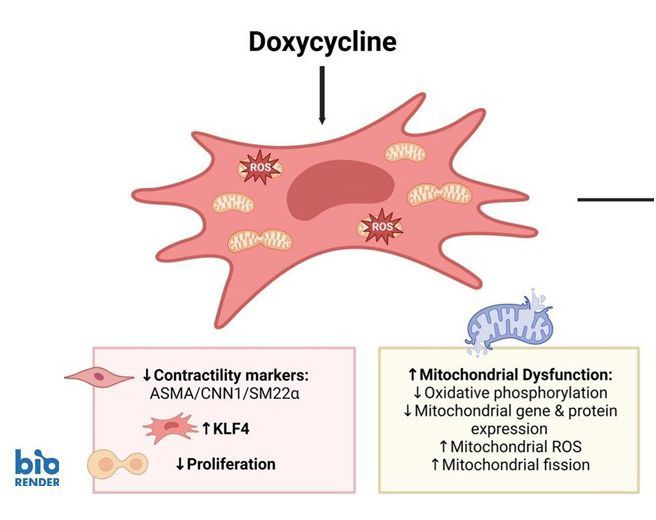
Doxycycline induces mitochondrial dysfunction in aortic smooth muscle cells
The antibiotic doxycycline is known to inhibit inflammation and was therefore considered as a therapeutic to prevent abdominal aortic aneurysm (AAA) growth. Yet mitochondrial dysfunction is a key-characteristic of clinical AAA disease. We hypothesize that doxycycline impairs mitochondrial function in the aorta and aortic smooth muscle cells (SMCs). Doxycycline induced mitonuclear imbalance, reduced proliferation and diminished expression of typical contractile smooth muscle cell (SMC) proteins. To understand the underlying mechanism, we studied krüppel-like factor 4 (KLF4). The expression of this transcription factor was enhanced in SMCs after doxycycline treatment. Knockdown of KLF4, however, did not affect the doxycycline-induced SMC phenotypic changes. Then we used the bioenergetics drug elamipretide (SS-31). Doxycycline-induced loss of SMC contractility markers was not rescued, but mitochondrial genes and mitochondrial connectivity improved upon elamipretide. Thus while doxycycline is anti-inflammatory, it also induces mitochondrial dysfunction in aortic SMCs and causes SMC phenotypic switching, potentially contributing to aortic aneurysm pathology. The drug elamipretide may be of interest for treatment of aneurysm diseases with pre-existing mitochondrial dysfunction.
De Waard Lab
The team
De Waard Lab
Alumni
Former PhD students:
Victorine Pinas
Anouk Hamers
Kondababu Kurakula
Goran Marinkovic
Stijntje Hibender
Romy Franken
Alexander den Hartog
Duco Koenis
Lejla Medzikovic
Lilian Schimmel
Maria Clemente-Olivo
Victoria Tedjawirja
Mitzi van Andel
Arnout Mieremet (postdoc)
Carmen Yap
de Waard Lab