De Winther Lab
Research
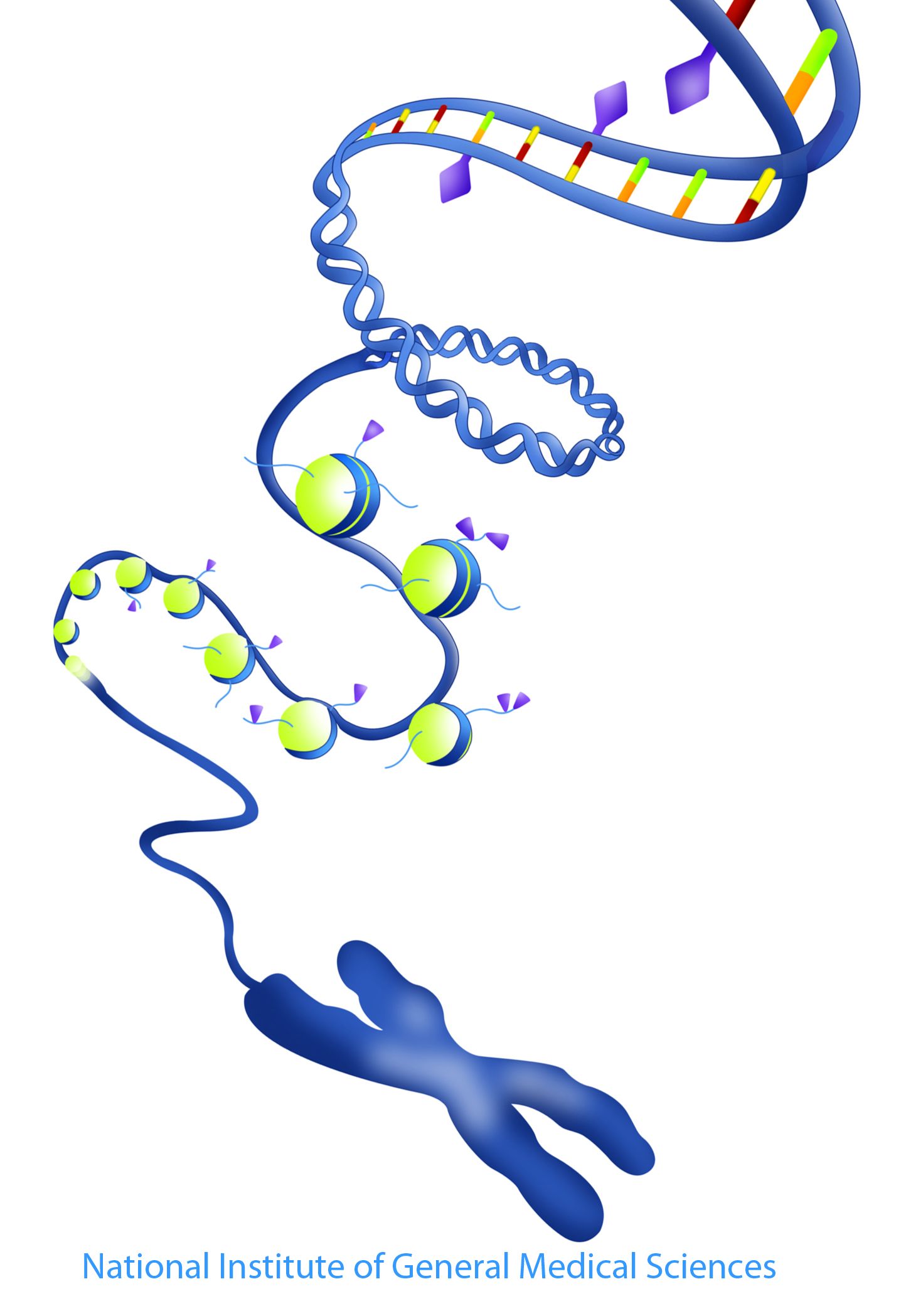
Epigenetics
It is increasingly being recognized that epigenetic processes (ie histone modifications, DNA methylation) are dynamically regulated in cells and critically control transcriptional responses in inflammation and immunity.
Our group is investigating the regulation of the epigenetic landscape of monocytes and macrophages in health and disease settings and is evaluating how interventions targeting epigenetic enzymes can be used to control inflammatory responses.
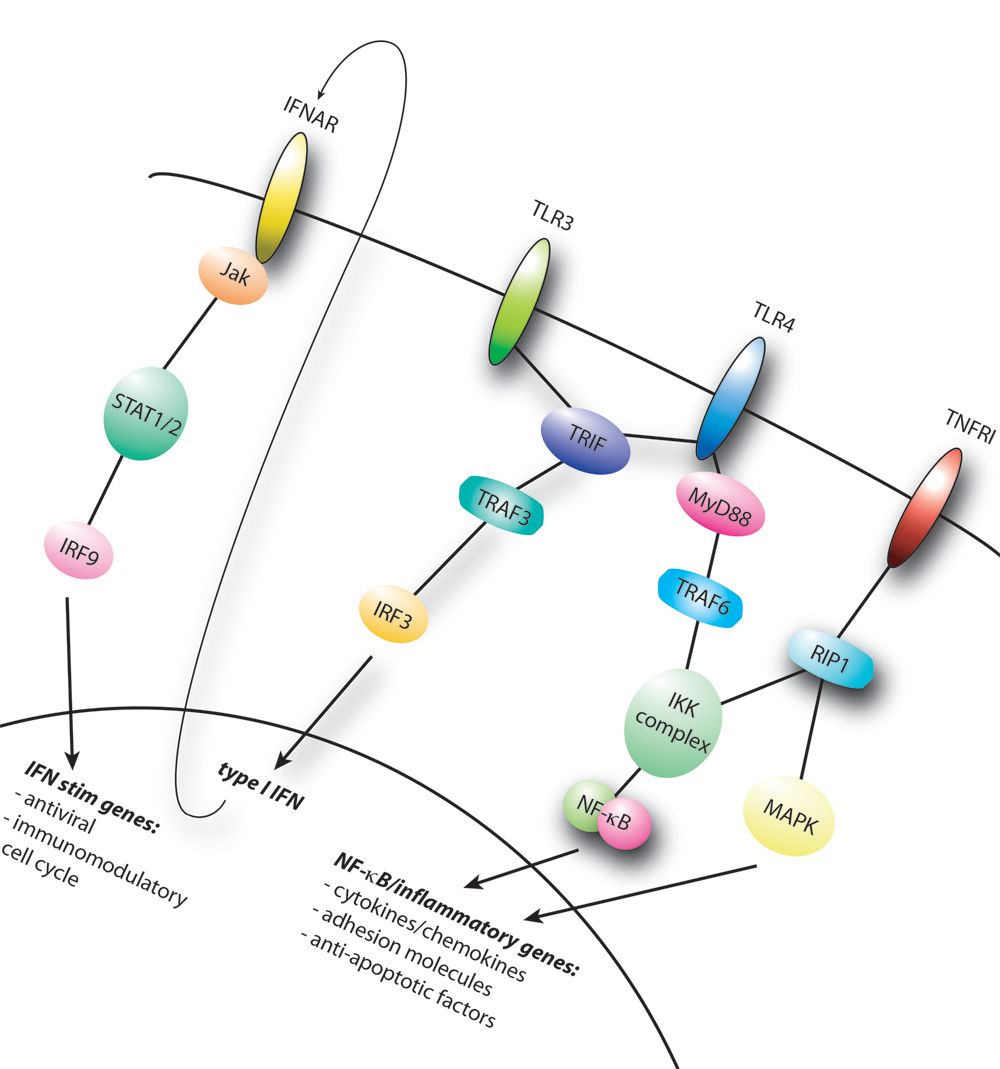
Cytokines and signaling pathways
Cytokines are key drivers in inflammation and immune responses. Through induction of specific signaling pathways they critically determine cellular responses and thereby dictate disease development. Cytokines such as IFNγ, IFNβ or TNF can skew macrophages towards pro-inflammatory, so-called M1, responses which are important for antiviral responses and bactericidal activity. Other cytokines such as IL-4 and IL-13 skew macrophages to a so called alternatively activated program which regulate fibrosis and wound healing. IL-10 is essential for general dampening of macrophage activation and is important for resolution of inflammation.
In our group we try to identify how we can manipulate cytokine responses and downstream signaling pathways to skew macrophages to a atherosclerosis-beneficial phenotype.
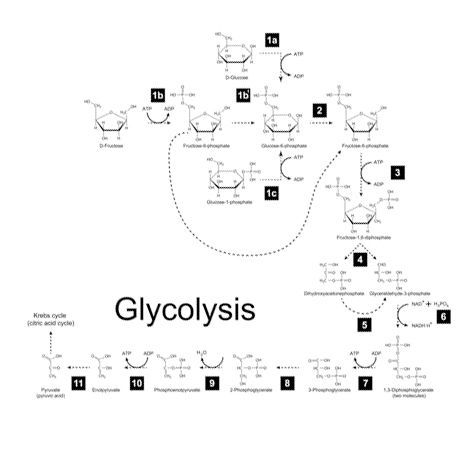
Immunometabolism
Recent data shows that cellular energy metabolism is strongly associated with macrophage inflammatory responses. While pro-inflammatory responses are driven by increased glycolysis, an anti-inflammatory macrophage thrives on oxidative phosphorylation. Specific interventions in glycolytic pathways or oxidative phosphorylation strongly influence macrophage activation patterns.
We are performing studies to identify metabolic characteristics that specify macrophage subtypes and interventions to modulate macrophage functioning in disease.
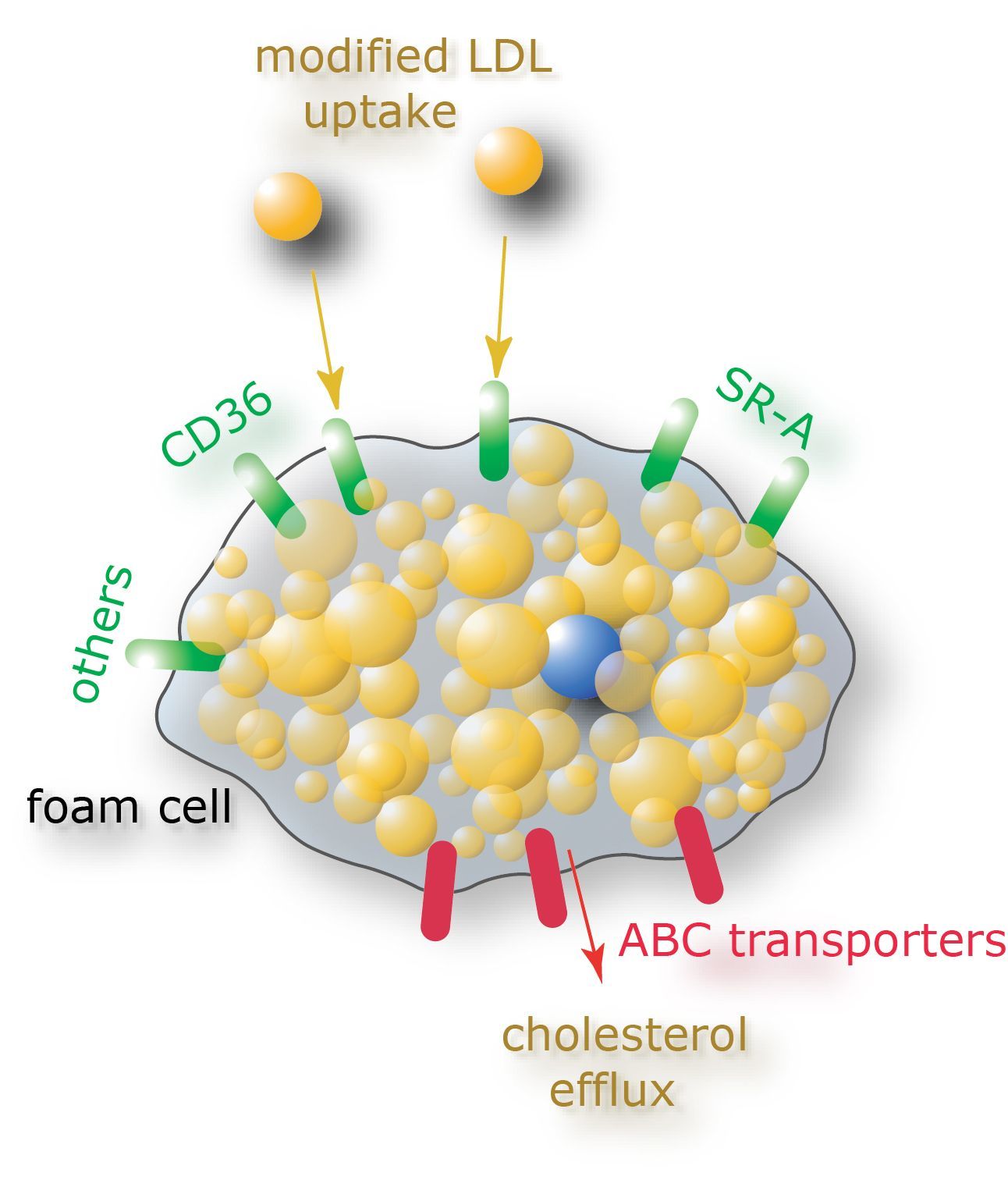
Hyperlipidemia
Atherosclerosis develops in a setting of hyperlipidemia, characterized by elevated levels of LDL- and VLDL-sized particles leading to increased plasma levels of cholesterol. A key hallmark of atherosclerosis is the intracellular accumulation of lipids in macrophages. This feature makes atherosclerosis a unique chronic inflammatory disease, since macrophages are thus functioning in a setting of dysbalanced intra- and extracellular lipid levels.
Our group is studying how lipids regulate macrophage responses in atherosclerosis and can be
used to control its behavior in disease.
De Winther Lab
The team
De Winther Lab
Photos/videos












